drugdevelopR: Optimal Phase II/III Drug Development Planning
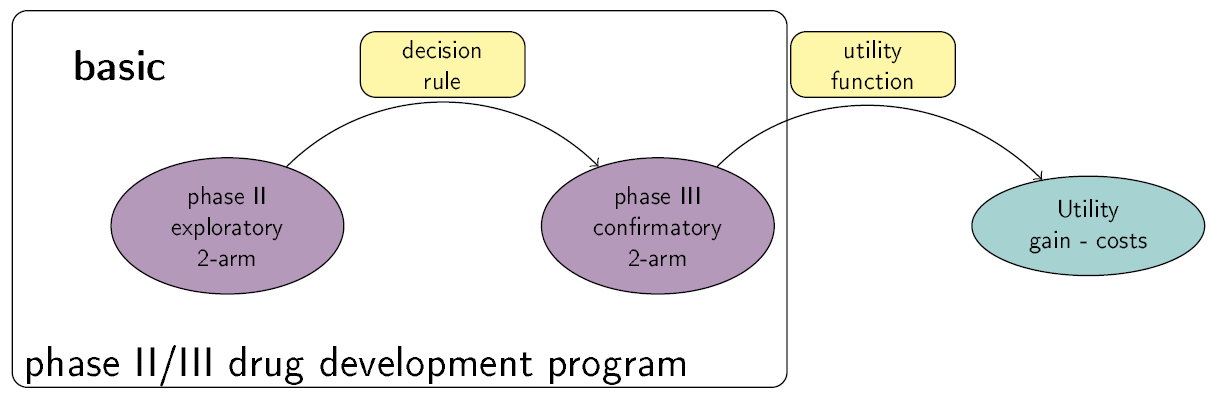
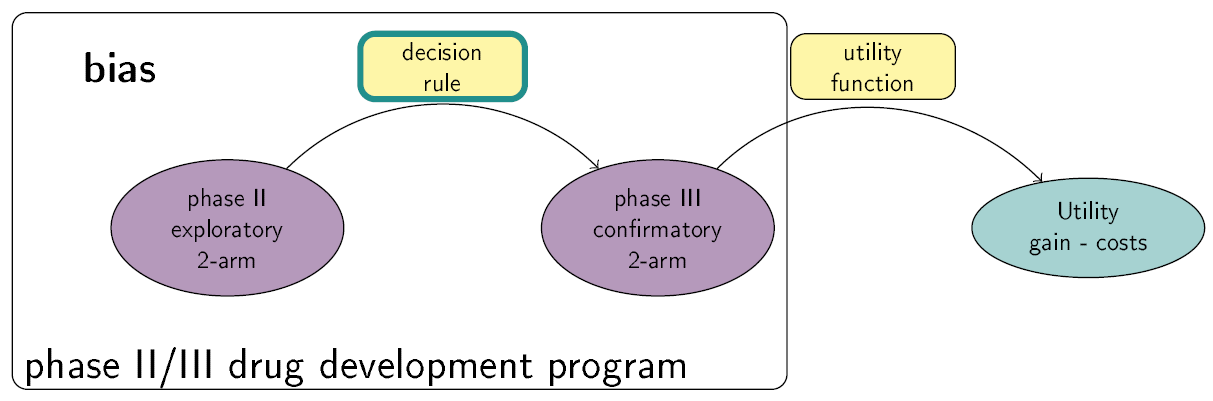
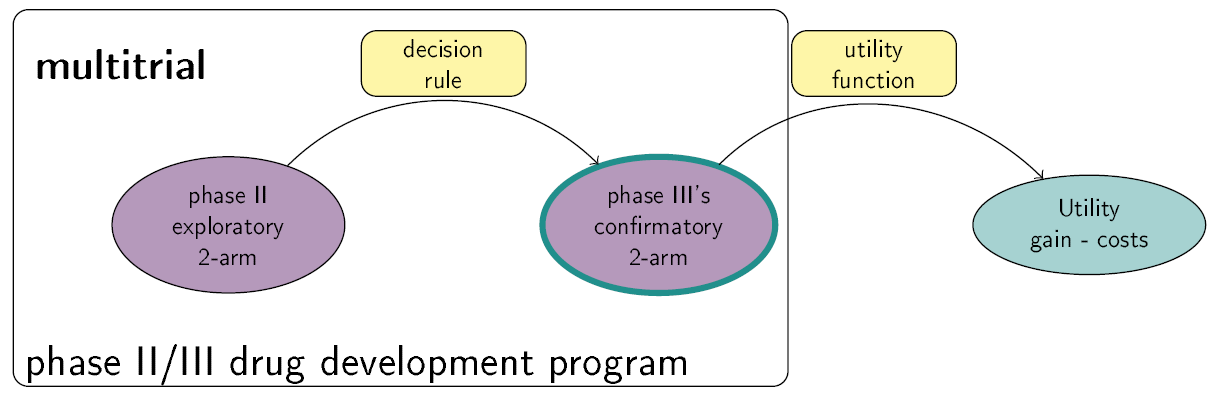
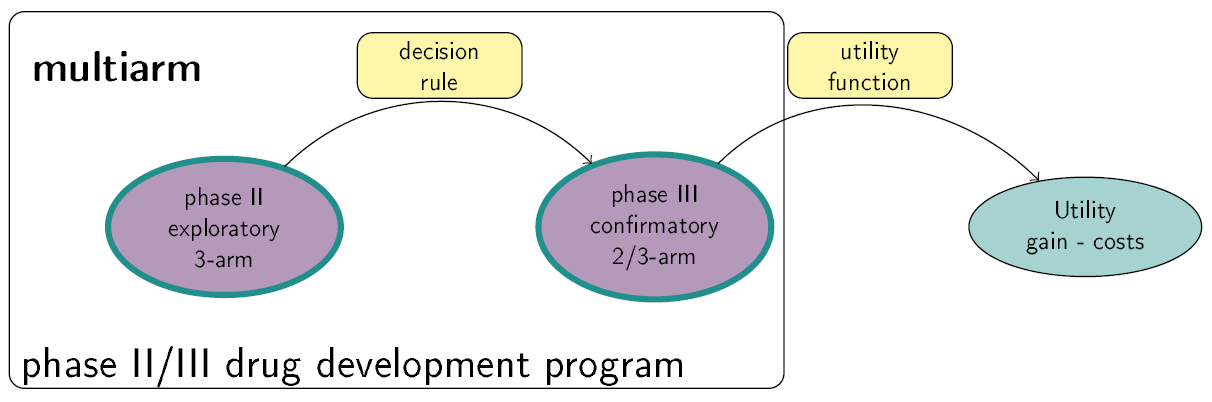
This Shiny App is a tool for phase II/III drug development planning aiming to optimize the sample size allocation and go/no-go decision rule with respect to the maximal expected utility. The utility function takes into account, e.g., costs of the program, expected benefits after successful market launch and assumed true treatment effects. Extensions to the basic framework include incorporating bias adjustment methods, multiple phase III trials and phase III trials with multiple arms. See also https://github.com/Sterniii3/drugdevelopR for the associated R package.
Maintainer
Stella Erdmann, Institute of Medical Biometry, University of Heidelberg, email: erdmann@imbi.uni-heidelberg.de.
Beta version 0.4